Durysta
About Durysta
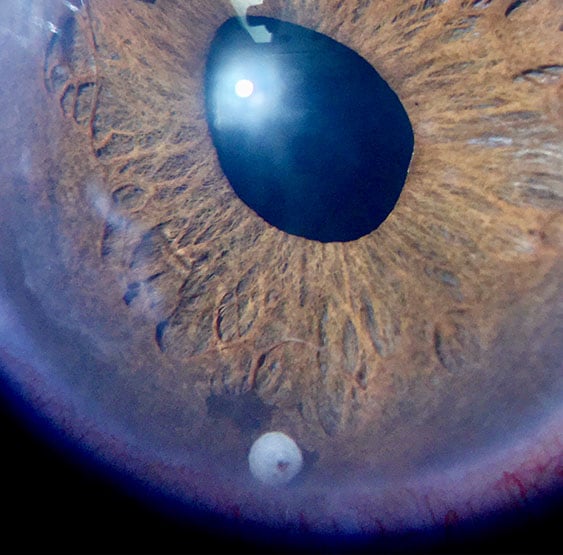
Durysta is a sustained release medication that is painlessly inserted in the front part of the eye to help reduce intraocular pressure (IOP). It is used to manage open-angle glaucoma or in patients with elevated IOP due to ocular hypertension.
The implant slowly releases bimatoprost over several months. Some patients may have the benefits of reducing the number of daily glaucoma drops or not having to use daily drops to manage their IOP at all.
Learn More About Durysta
Schedule now by completing this form or calling us at (850) 331-3937 to discuss with Dr. Phil Alabata if you are a candidate for Durysta. A referral is not required.
